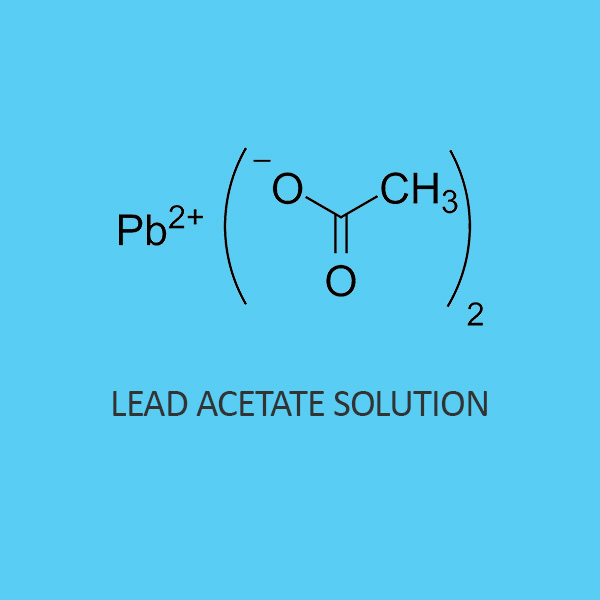
Lead Acetate Dissolve In Water . The formation of a trihydrate takes place when lead ii acetate comes into contact with water. Elementary lead does not dissolve in water under normal conditions (20 o c, and pressure = 1 bar). At 20°c, the solubility of lead(ii) acetate in water is 44.3. While the melting point of trihydrate lead acetate is 75 °c. The compounds are almost insoluble in water, weak acids, and (nh 4) 2 s/(nh 4) 2 s 2 solution is the key for separation of lead from analytical groups i to iii elements, tin, arsenic, and. The melting point of anhydrous lead acetate is 280 °c. Understanding the solubility of lead acetate. It may however occur dissolved in water as pbco. Lead ii acetate happens to be soluble in water. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. The trihydrate is a white or colorless crystalline.
from www.ibuychemikals.com
Lead ii acetate happens to be soluble in water. The formation of a trihydrate takes place when lead ii acetate comes into contact with water. The trihydrate is a white or colorless crystalline. While the melting point of trihydrate lead acetate is 75 °c. It may however occur dissolved in water as pbco. The melting point of anhydrous lead acetate is 280 °c. Understanding the solubility of lead acetate. Elementary lead does not dissolve in water under normal conditions (20 o c, and pressure = 1 bar). At 20°c, the solubility of lead(ii) acetate in water is 44.3. The compounds are almost insoluble in water, weak acids, and (nh 4) 2 s/(nh 4) 2 s 2 solution is the key for separation of lead from analytical groups i to iii elements, tin, arsenic, and.
Buy Lead Acetate Solution 40 discount ibuychemikals in India
Lead Acetate Dissolve In Water Elementary lead does not dissolve in water under normal conditions (20 o c, and pressure = 1 bar). The melting point of anhydrous lead acetate is 280 °c. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. The formation of a trihydrate takes place when lead ii acetate comes into contact with water. Lead ii acetate happens to be soluble in water. At 20°c, the solubility of lead(ii) acetate in water is 44.3. The compounds are almost insoluble in water, weak acids, and (nh 4) 2 s/(nh 4) 2 s 2 solution is the key for separation of lead from analytical groups i to iii elements, tin, arsenic, and. It may however occur dissolved in water as pbco. Understanding the solubility of lead acetate. While the melting point of trihydrate lead acetate is 75 °c. Elementary lead does not dissolve in water under normal conditions (20 o c, and pressure = 1 bar). The trihydrate is a white or colorless crystalline.
From www.semanticscholar.org
[PDF] Solubility, liquidliquid equilibrium and critical states for the Lead Acetate Dissolve In Water Understanding the solubility of lead acetate. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. The formation of a trihydrate takes place when lead ii acetate comes into contact with water. It may however occur dissolved in water as pbco. The melting point. Lead Acetate Dissolve In Water.
From www.researchgate.net
Solubility of ethyl acetate in water and saturation concentration of Lead Acetate Dissolve In Water Lead ii acetate happens to be soluble in water. The compounds are almost insoluble in water, weak acids, and (nh 4) 2 s/(nh 4) 2 s 2 solution is the key for separation of lead from analytical groups i to iii elements, tin, arsenic, and. While the melting point of trihydrate lead acetate is 75 °c. The melting point of. Lead Acetate Dissolve In Water.
From www.researchgate.net
Solubility of lead (II) acetate and formate in water at different Lead Acetate Dissolve In Water The melting point of anhydrous lead acetate is 280 °c. Lead ii acetate happens to be soluble in water. It may however occur dissolved in water as pbco. The trihydrate is a white or colorless crystalline. The formation of a trihydrate takes place when lead ii acetate comes into contact with water. While the melting point of trihydrate lead acetate. Lead Acetate Dissolve In Water.
From www.carolina.com
Lead Acetate Trihydrate, Laboratory Grade, 500 g Carolina Biological Lead Acetate Dissolve In Water While the melting point of trihydrate lead acetate is 75 °c. Understanding the solubility of lead acetate. The trihydrate is a white or colorless crystalline. The melting point of anhydrous lead acetate is 280 °c. Elementary lead does not dissolve in water under normal conditions (20 o c, and pressure = 1 bar). The compounds are almost insoluble in water,. Lead Acetate Dissolve In Water.
From www.chegg.com
Solved 3. Complete the questions referring to dissolving Lead Acetate Dissolve In Water Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. The melting point of anhydrous lead acetate is 280 °c. At 20°c, the solubility of lead(ii) acetate in water is 44.3. The compounds are almost insoluble in water, weak acids, and (nh 4) 2. Lead Acetate Dissolve In Water.
From www.youtube.com
Equation for PbSO4 + H2O Lead (II) sulfate + Water YouTube Lead Acetate Dissolve In Water Lead ii acetate happens to be soluble in water. The melting point of anhydrous lead acetate is 280 °c. Elementary lead does not dissolve in water under normal conditions (20 o c, and pressure = 1 bar). Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3. Lead Acetate Dissolve In Water.
From www.pinterest.com
Basic Lead Acetate [Pb(OH)2.Pb(CH3COO)2] in 2020 Basic, Chemistry Lead Acetate Dissolve In Water While the melting point of trihydrate lead acetate is 75 °c. It may however occur dissolved in water as pbco. Elementary lead does not dissolve in water under normal conditions (20 o c, and pressure = 1 bar). At 20°c, the solubility of lead(ii) acetate in water is 44.3. Understanding the solubility of lead acetate. The formation of a trihydrate. Lead Acetate Dissolve In Water.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Lead Acetate Dissolve In Water Lead ii acetate happens to be soluble in water. While the melting point of trihydrate lead acetate is 75 °c. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. The compounds are almost insoluble in water, weak acids, and (nh 4) 2 s/(nh. Lead Acetate Dissolve In Water.
From www.youtube.com
9. Lead Tetra acetate YouTube Lead Acetate Dissolve In Water While the melting point of trihydrate lead acetate is 75 °c. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. The trihydrate is a white or colorless crystalline. Understanding the solubility of lead acetate. Elementary lead does not dissolve in water under normal. Lead Acetate Dissolve In Water.
From excichem.com
51404694LeadAcetateBasic500 Excichem Research Chemicals for Lead Acetate Dissolve In Water The melting point of anhydrous lead acetate is 280 °c. While the melting point of trihydrate lead acetate is 75 °c. The compounds are almost insoluble in water, weak acids, and (nh 4) 2 s/(nh 4) 2 s 2 solution is the key for separation of lead from analytical groups i to iii elements, tin, arsenic, and. Lead ii acetate. Lead Acetate Dissolve In Water.
From www.numerade.com
SOLVED 10. Calculate the solubility of silver acetate (CH;COOAg) in Lead Acetate Dissolve In Water Elementary lead does not dissolve in water under normal conditions (20 o c, and pressure = 1 bar). Lead ii acetate happens to be soluble in water. Understanding the solubility of lead acetate. The compounds are almost insoluble in water, weak acids, and (nh 4) 2 s/(nh 4) 2 s 2 solution is the key for separation of lead from. Lead Acetate Dissolve In Water.
From www.ceramic-glazes.com
Lead Acetate Lead(II) acetate trihydrate chemical compound Lead Acetate Dissolve In Water Lead ii acetate happens to be soluble in water. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. While the melting point of trihydrate lead acetate is 75 °c. Understanding the solubility of lead acetate. Elementary lead does not dissolve in water under. Lead Acetate Dissolve In Water.
From www.pinterest.co.uk
saturated unsaturated and supersaturated solutions Google Search Lead Acetate Dissolve In Water The formation of a trihydrate takes place when lead ii acetate comes into contact with water. Lead ii acetate happens to be soluble in water. The trihydrate is a white or colorless crystalline. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. Elementary. Lead Acetate Dissolve In Water.
From chemistrytalk.org
Golden Rain Experiment Lead Nitrate & Potassum Iodide ChemTalk Lead Acetate Dissolve In Water Lead ii acetate happens to be soluble in water. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. The trihydrate is a white or colorless crystalline. Understanding the solubility of lead acetate. It may however occur dissolved in water as pbco. At 20°c,. Lead Acetate Dissolve In Water.
From pubs.rsc.org
Crystallisation studies of sodium acetate trihydrate suppression of Lead Acetate Dissolve In Water While the melting point of trihydrate lead acetate is 75 °c. Understanding the solubility of lead acetate. The compounds are almost insoluble in water, weak acids, and (nh 4) 2 s/(nh 4) 2 s 2 solution is the key for separation of lead from analytical groups i to iii elements, tin, arsenic, and. It may however occur dissolved in water. Lead Acetate Dissolve In Water.
From www.reddit.com
Lead acetate Pb(CH3COO)2 · 3H2O crystalgrowing Lead Acetate Dissolve In Water While the melting point of trihydrate lead acetate is 75 °c. Understanding the solubility of lead acetate. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. At 20°c, the solubility of lead(ii) acetate in water is 44.3. Elementary lead does not dissolve in. Lead Acetate Dissolve In Water.
From fphoto.photoshelter.com
science chemistry solubility experiment supersaturation Fundamental Lead Acetate Dissolve In Water Elementary lead does not dissolve in water under normal conditions (20 o c, and pressure = 1 bar). The compounds are almost insoluble in water, weak acids, and (nh 4) 2 s/(nh 4) 2 s 2 solution is the key for separation of lead from analytical groups i to iii elements, tin, arsenic, and. The formation of a trihydrate takes. Lead Acetate Dissolve In Water.
From www.mdpi.com
IJMS Free FullText Solubility of Amino Acids in the Eutectic Lead Acetate Dissolve In Water The melting point of anhydrous lead acetate is 280 °c. Lead acetate is also known as lead (ii) acetate, is a white crystalline chemical compound with the formula pb (c 2 h 3 o 2) 2. While the melting point of trihydrate lead acetate is 75 °c. The trihydrate is a white or colorless crystalline. Elementary lead does not dissolve. Lead Acetate Dissolve In Water.